[vc_row][vc_column][vc_column_text]
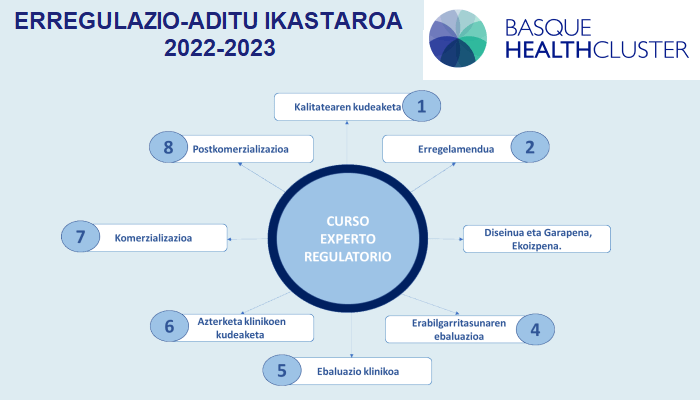
With the class on 2 December, we have completed module 6 of the “Regulatory Expert” course organised by the Basque Health Cluster. After a Christmas break, we will start on 13 January with module 7, which forms part of the third and final part.
Basque Health Cluster, under the Regulations (EU) 2017/745 and 2017/746 of the European Parliament and of the Council of 5 April 2017, has organised this intensive course on the analysis and understanding of the new regulations affecting medical devices (MPs) and in vitro diagnostic (IVD) medical devices. This course, launched at the beginning of the year, aims to provide bio-health professionals with the knowledge, skills and competencies necessary to adapt both MDR and IVDR to the new regulations.
In this third part we will have the following teachers who will instruct us in “Commercialisation”, “Post-marketing” and EUDAMED:
The methodology of the course will combine theory and practice through lectures and group workshops. Classes will be held in person on Fridays from 09:30 to 13:30 at the Basque Health Cluster headquarters (BIC Bizkaia: Camino Astondo, building 612, 48160 Derio, Parque Científico y Tecnológico de Bizkaia).
[/vc_column_text][vc_column_text]
There is the possibility of registering for the modules independently without taking the whole course. The M7 and M8 module and the final module are currently open. The registration deadlines and prices are shown in the following table:
|
Module |
Enrolment closes |
Price for members | Price for non-members |
|
Module 7 (Marketing) |
January 6 |
150 € + VAT |
325 € + VAT |
|
Module 8 (Post-marketing) |
January 20 |
150 € + IVA |
325 € + IVA |
|
Last Module |
February 3 |
75 € + IVA |
162 € + IVA |
For more information and to download the program (in Spanish) click on the following button:
[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column][vc_btn title=”Programme” style=”outline-custom” outline_custom_color=”#9595c9″ outline_custom_hover_background=”#9595c9″ outline_custom_hover_text=”#ffffff” i_icon_fontawesome=”fas fa-edit” add_icon=”true” link=”url:https%3A%2F%2Fbasquehealthcluster.org%2Fwp-content%2Fuploads%2F2022%2F03%2FCurso-Experto-en-Regulatoria_BHC_-Castellano.pdf|target:_blank”][vc_column_text]
In order to enroll click on the following button:
[/vc_column_text][vc_btn title=”Sign in” style=”outline-custom” outline_custom_color=”#9595c9″ outline_custom_hover_background=”#9595c9″ outline_custom_hover_text=”#ffffff” align=”center” i_icon_fontawesome=”fas fa-edit” add_icon=”true” link=”url:https%3A%2F%2Fforms.gle%2F9Xsqo3MouaAhdBAT7|target:_blank”][/vc_column][/vc_row]