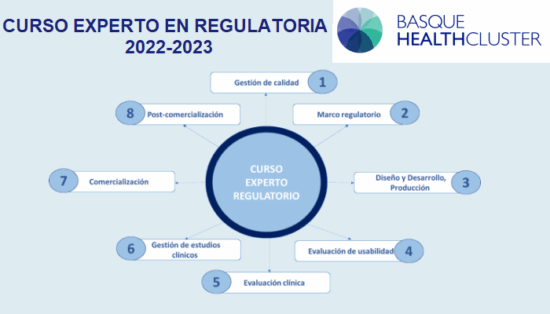
[vc_row][vc_column][vc_column_text]After finishing the second module on 24 June and taking a holiday period, the “Expert on Regulatory Affairs” course starts on 9 September. Every Friday, until 2...


| Cookie | Type | Duration | Description |
|---|---|---|---|
| cookielawinfo-checkbox-necessary | persistent | 11 months | This cookie is set by the GDPR Cookie Consent plugin. It is used to store user consent for the cookies in the “Necessary” category. |
| cookielawinfo-checkbox-non-necessary | persistent | 11 months | This cookie is set by the GDPR Cookie Consent plugin. It is used to store user consent for the cookies in the “Non-Necessary” category. |
| viewed_cookie_policy | persistent | 11 months | This cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not the user has consented to the use of cookies. It does not store any personal data. |
| Cookie | Type | Duration | Description |
|---|---|---|---|
| __utma | persistent | 2 years | This cookie is set by Google Analytics and is used to distinguish users and sessions. It is created when the JavaScript library executes and there are no existing __utma cookies. It is updated every time data is sent to Google Analytics. |
| __utmb | persistent | 30 minutes | This cookie is set by Google Analytics. It is used to determine new sessions/visits. It is created when the JavaScript library executes and there are no existing __utma cookies. It is updated every time data is sent to Google Analytics. |
| __utmc | session | End of browser session | This cookie is set by Google Analytics and is deleted when the user closes their browser. It is not used by ga.js. It is used to enable interoperability with urchin.js, which is an older version of Google Analytics and used in conjunction with the __utmb cookie to determine new sessions/visits. |
| __utmt | persistent | 10 minutes | This cookie is set by Google Analytics and used to throttle the request rate. |
| __utmz | persistent | 6 months | This cookie is set by Google Analytics and used to store the traffic source or campaign through which the visitor reaches the site. |
| _ga | persistent | 2 years | This cookie is used to distinguish users and sessions. |
| _gid | persistent | 1 day | This cookie is used to distinguish users and sessions. |
| GPS | persistent | 30 minutes | This cookie is set by YouTube and registers a unique ID for tracking users based on their geographical location. |
| test_cookie | persistent | 11 months | Google DoubleClick can set this cookie on our website to check whether the user’s browser accepts cookies. Google DoubleClick aims to improve the advertising the user sees. |
| YSC | session | End of browser session | This cookie is set by YouTube and is used to track views of embedded videos. |
| Cookie | Type | Duration | Description |
|---|---|---|---|
| IDE | persistent | 2 years | This cookie is used by Google DoubleClick and stores information about how the user interacts with the website and any other advert before visiting the website. This data is used to present the user with adverts that are relevant to them based on their user profile. |
| uid | persistent | 1 month | This cookie is used to anonymously measure the number and behaviour of visitors to the website. This data includes the number of visits, average visit duration, pages visited, etc. to better understand user preferences for targeted adverts. |
| VISITOR_INFO1_LIVE | persistent | 5 months | This cookie is set by YouTube. It is used to track information for YouTube videos embedded on a website. |
| Cookie | Type | Duration | Description |
|---|---|---|---|
| lang | persistent | 1 year | This cookie is used to store the language preferences of a user to serve up content in that stored language the next time user visit the website. |
| YTC | persistent | 10 minutes | This cookie is set by YouTube to manage the embedding and viewing of videos on our website. |